Canavan disease is an extremely rare genetic disease that begins in infancy and progresses rapidly. While symptoms vary, many patients experience lack of head control, lack of muscle tone (often resulting in floppiness or spasticity) and seizures. Most children are not able to meet developmental milestones, and are unable to crawl, walk, sit or talk. Many pass away before the age of 20.
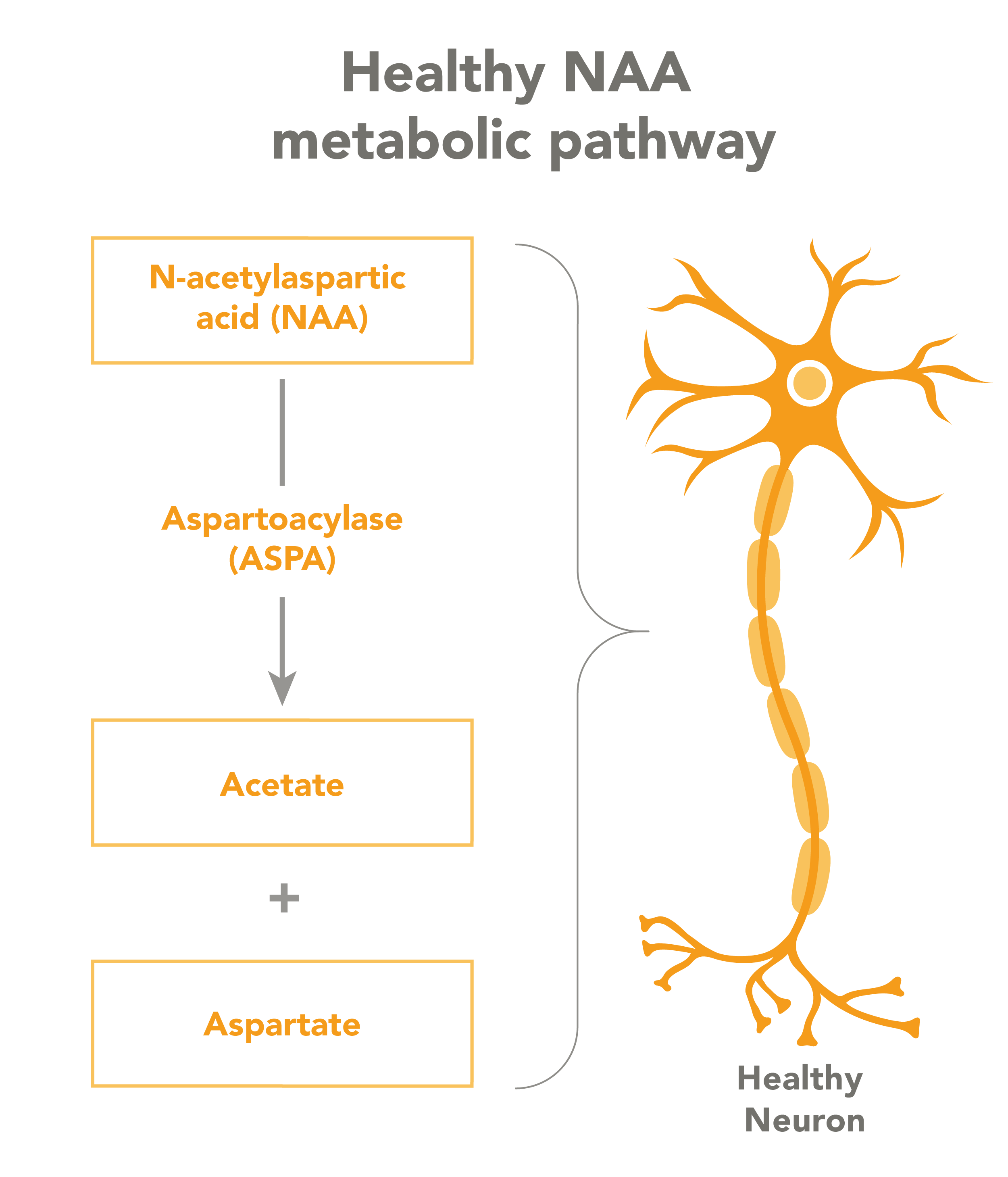
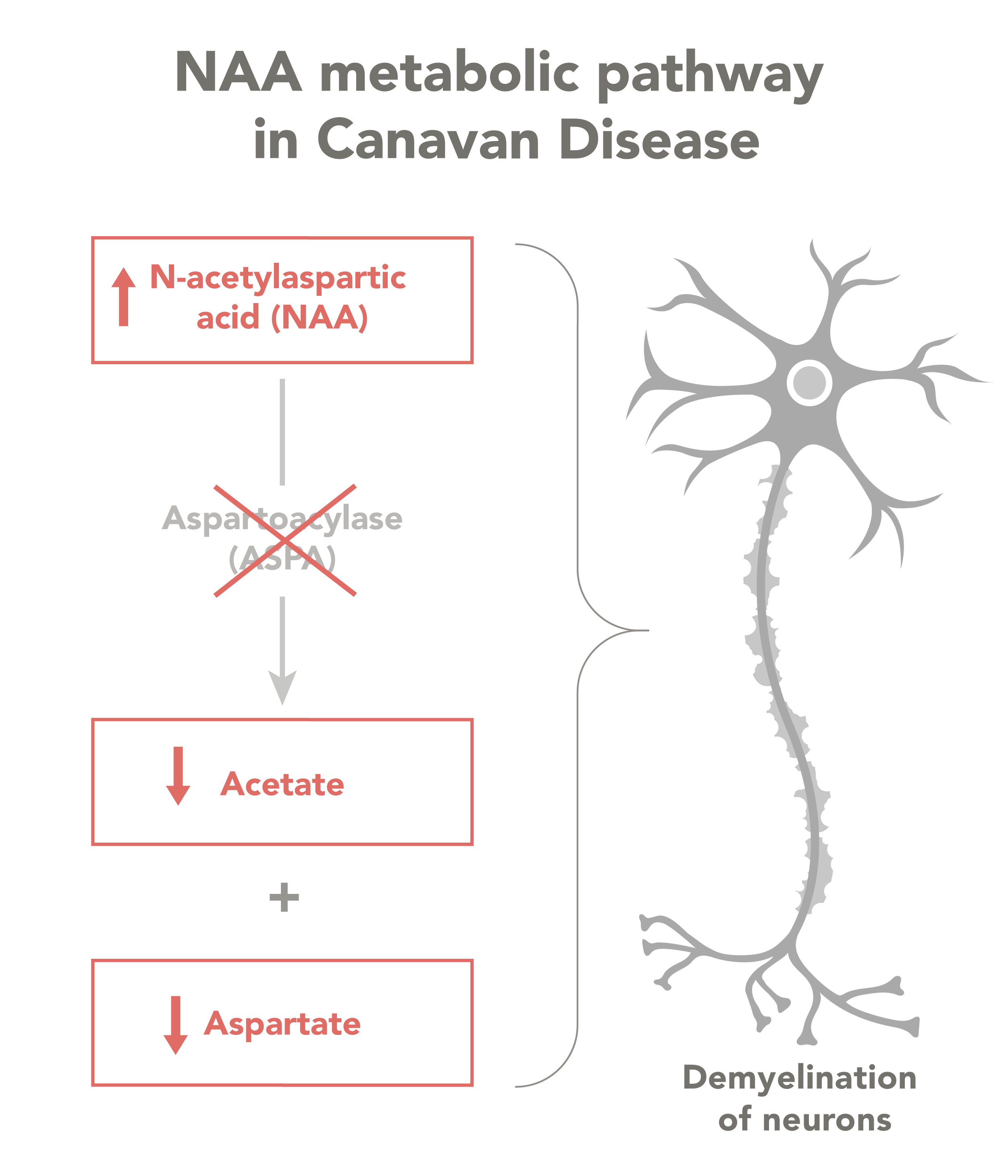
The disease is caused by an inherited mutation of the ASPA gene, which codes for a protein that breaks down NAA. When NAA levels get too high, it becomes toxic to myelin in ways that are not well understood. Myelin insulates the nerves, and without it, neurons are unable to send and receive messages as they should. The condition also affects important metabolic processes.
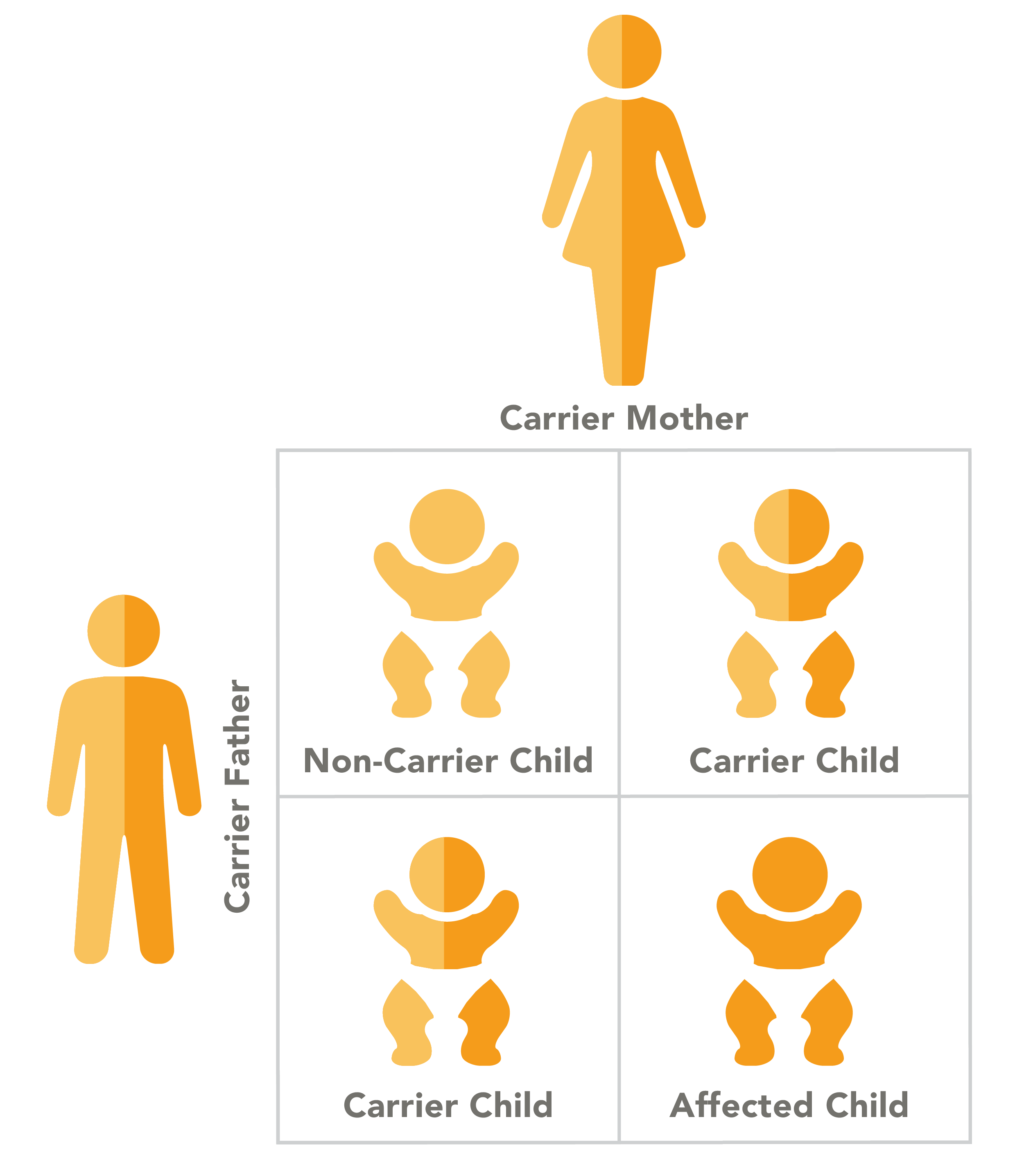
Canavan disease has an autosomal recessive pattern of inheritance, which means that a child with the disease inherited a copy of the mutated gene from each parent. In autosomal recessive conditions, parents are carriers of the gene, but generally do not experience the symptoms of the disease.
Currently, there are no approved therapies for Canavan disease. The current standard of care is limited to supportive therapy. Aspa Therapeutics has partnered with the University of Massachusetts Medical School to advance their gene therapy program for Canavan disease and pursue a clinical trial.